Articles:
phosphoric acid, calcium salt (2:1)
Notes:
mw 234.05. Added to flour as a nutrient source and leavening agent; stabiliser and thickener for fruit jellies, preserves and jams; also used in foods as pH control agent, firming agent, sequestrant and dietary supplement
| CAS Number: | 7758-23-8 | 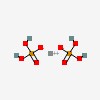 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 231-837-1 | |
| FDA UNII: | 701EKV9RMN | |
| MDL: | MFCD00149602 | |
| Molecular Weight: | 234.05100400 | |
| Formula: | Ca H4 O8 P2 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: buffering, firming, sequestrant, leavening, dough conditioner, texturizer, yeast food, nutrient, acidity regulators
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Appearance: | white crystalline granules (est) |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 157.00 to 158.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 1.410000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| logP (o/w): | -2.148 (est) |
| Soluble in: | |
| water, slightly | |
| water, 1e+006 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| alcohol | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
buffering agents |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/37/38 - Irritating to eyes, respiratory system, and skin. S 02 - Keep out of the reach of children. S 22 - Do not breath dust. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 3986 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE SKIN AND APPENDAGES (SKIN): HAIR: OTHER National Technical Information Service. Vol. OTS0571950 oral-mouse LD50 15250 mg/kg Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 87, 1987. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 2000 mg/kg SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" National Technical Information Service. Vol. OTS0571950 | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | buffering, firming, sequestrant, leavening, dough conditioner, texturizer, yeast food, nutrient, acidity regulators | ||
| Recommendation for calcium phosphate monobasic usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for calcium phosphate monobasic flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Assessment of one published review on health risks associated with phosphate additives in food View page or View pdf | |
| Re-evaluation of phosphoric acid–phosphates – di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 7758-23-8 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 24454 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| calcium dihydrogen phosphate | |
| Chemidplus: | 0007758238 |
| RTECS: | TB8527000 for cas# 7758-23-8 |
References:
| calcium dihydrogen phosphate | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 7758-23-8 |
| Pubchem (cid): | 24454 |
| Pubchem (sid): | 134989991 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| CHEBI: | View |
| KEGG (GenomeNet): | C13556 |
| HMDB (The Human Metabolome Database): | Search |
| FooDB: | FDB013367 |
| Export Tariff Code: | 2835.26.0000 |
| FDA Listing of Food Additive Status: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •superphosphate obtained from sulfuric acid treatment is about 30% calcium superphosphate, 10% dicalcium orthophosphate, 45% calcium sulfate, 10% iron oxide, silica, alumina et cetera, & 5% water contains 18-21% avail phosphorus pentoxide. triple superphosphate from phosphoric acid treatment contains from 43-50% avail phosphorus pentoxide. •grade: fcc food chemicals codex, ceramic, anhydrous, hydrated | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| abrasives | ||
| buffering agents | ||
| oral care agents | ||
| viscosity controlling agents |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| acid calcium phosphate | |
| calcium biphosphate | |
| calcium bis(dihydrogen phosphate) | |
| calcium bis(dihydrogenphosphate) | |
| calcium dihydrogen phosphate | |
| calcium dihydrogenphoshate | |
| calcium dihydrogenphosphate | |
| calcium hydrogen phosphate (Ca(H2PO4)2) | |
| calcium monobasic phosphate | |
| mono | calcium orthophosphate |
| mono | calcium phosphate |
| calcium phosphate monobasic | |
| calcium phosphate, monobasic | |
| mono | calcium phosphate, monobasic |
| calcium tetrahydrogen diphosphate | |
| calciumdihydrogenorthophosphate | |
| calciumphosphatemonobasic | |
| phosphoric acid, calcium salt (2:1) |


