Articles:
adenine, N6-benzyl-
Notes:
6-Benzylaminopurine, benzyl adenine or BAP is a first-generation synthetic cytokinin which elicits plant growth and development responses, setting blossoms and stimulating fruit richness by stimulating cell division. It is an inhibitor of respiratory kinase in plants, and increases post-harvest life of green vegetables. (Wikipedia)
| CAS Number: | 1214-39-7 | 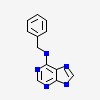 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 124786-41-0 | |
| ECHA EINECS - REACH Pre-Reg: | 214-927-5 | |
| FDA UNII: | KXG6A989PS | |
| Nikkaji Web: | J1.764G | |
| Beilstein Number: | 616790 | |
| MDL: | MFCD00005572 | |
| XlogP3: | 2.10 (est) | |
| Molecular Weight: | 225.25467000 | |
| Formula: | C12 H11 N5 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: cosmetic ingredient for hair conditioning
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | colorless fine needles (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 233.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 2.373000 mmHg @ 20.00 °C. (est) |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| logP (o/w): | 1.570 |
| Soluble in: | |
| water, 60 mg/L @ 20 °C (exp) | |
| water, 1472 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
hair conditioning |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| 6-Benzylaminopurine 95%
Odor: characteristic Use: 6-Benzylaminopurine, a purine-based first-generation synthetic cytokinin, could stimulate plant growth.
6-Benzylaminopurine is a purine-based first-generation synthetic cytokinin that could stimulate plant growth. |
| EMD Millipore |
| For experimental / research use only. |
| 6-Benzylaminopurine |
| Glentham Life Sciences |
| 6-Benzylaminopurine |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| 6-Benzylaminopurine ≥99% |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| 6-Benzylaminopurine ReagentPlus®, ≥99.0% (HPLC) |
| TCI AMERICA |
| For experimental / research use only. |
| N6-Benzyladenine >99.0%(HPLC)(T) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-mouse LD50 1300 mg/kg SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. oral-rat LD50 2125 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. | |
| Dermal Toxicity: | |
|
skin-mouse LD50 > 5000 mg/kg Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. subcutaneous-mouse LD50 > 2300 mg/kg Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic ingredient for hair conditioning | ||
| Recommendation for benzyladenine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for benzyladenine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Retrospective analysis of the immunotoxic effects of plant protection products as reported in the Draft Assessment Reports for their peer review at EU level View page or View pdf | |
| Review of the existing maximum residue levels for 6-benzyladenine according to Article 12 of Regulation (EC) No 396/2005 View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 1214-39-7 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 62389 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| N-benzyl-7H-purin-6-amine | |
| Chemidplus: | 0001214397 |
| RTECS: | AU6252200 for cas# 1214-39-7 |
References:
| N-benzyl-7H-purin-6-amine | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 1214-39-7 |
| Pubchem (cid): | 62389 |
| Pubchem (sid): | 135020588 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| KEGG (GenomeNet): | C11263 |
| HMDB (The Human Metabolome Database): | HMDB39238 |
| FooDB: | FDB018775 |
| Export Tariff Code: | 2933.90.8300 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •accel plant growth regulator solution: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 0.18% gibberellin a4 mixt. with gibberellin a7 •agtrol 6-b: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 0.18% gibberellin a4 mixt. with gibberellin a7 •6-benzyladenine technical (2 products): active ingredient 99.0 and 97.90% n-(phenylmethyl)-1h-purin-6-amine •ba6 technical: active ingredient 98.2% n-(phenylmethyl)-1h-purin-6-amine •bap-technical: active ingredient 98.80% n-(phenylmethyl)-1h-purin-6-amine •chrysal bvb: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 1.80% gibberellin a4 mixt. with gibberellin a7 •configure and exilis plus: active ingredient 2.00% n-(phenylmethyl)-1h-purin-6-amine •lt biosyn 6-benzylaminopurine technical: active ingredient 99.0% n-(phenylmethyl)-1h-purin-6-amine •maxcel: active ingredient 1.90% n-(phenylmethyl)-1h-purin-6-amine •perlan: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 1.80% gibberellin a4 mixt. with gibberellin a7 •promalin plant growth regulator solution: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 1.80% gibberellin a4 mixt. with gibberellin a7 •riteway: active ingredient 1.90% n-(phenylmethyl)-1h-purin-6-amine •typy plant growth regulator: active ingredients 1.80% n-(phenylmethyl)-1h-purin-6-amine and 1.80% gibberellin a4 mixt. with gibberellin a7 •paste •6-benzylaminopurine technical •water soluble concentrate | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| celery seed Search Trop Picture | |
| not found in nature | |
| tomato Search Trop Picture |
Synonyms:
| adenine, N-benzyl- | |
| adenine, N(sup 6)-benzyl- | |
| adenine, N6-benzyl- | |
| aminopurine, 6-benzyl | |
| benzyl adenine | |
| 6- | benzyl adenine |
| 6- | benzyl aminopurine |
| N- | benzyl-1H-purin-6-amine |
| N- | benzyl-3H-purin-6-amine |
| N- | benzyl-7H-purin-6-amine |
| N- | benzyl-9H-purin-6-amine |
| N6- | benzyl-9H-purin-6-amine |
| N- | benzyl-9H-purin-6-amine, N-(9H-Purin-6-yl)benzylamine |
| benzyl(purin-6-yl)amine | |
| 6-(N- | benzyl)aminopurine |
| 6- | benzyladenine |
| N-6- | benzyladenine |
| N(Sup6)- | benzyladenine |
| N6- | benzyladenine |
| N- | benzyladenine, 8CI |
| 6-( | benzylamino)-9H-purine |
| 6-( | benzylamino)purine |
| 6-(N- | benzylamino)purine |
| benzylaminopurine | |
| 6- | benzylaminopurine |
| benzylpurin-6-ylamine | |
| paturyl | |
| N-( | phenylmethyl)-1H-purin-6-amine |
| N-( | phenylmethyl)-1H-purine-6-amine |
| N-( | phenylmethyl)-9H-purin-6-amine |
| 6-[( | phenylmethyl)amino]-9H-purine |
| promalin | |
| 1H- | purin-6-amine, N-(phenylmethyl)- |
| 7H- | purin-6-amine, N-(phenylmethyl)- |
| 9H- | purin-6-amine, N-(phenylmethyl)- |
| N-(9H- | purin-6-yl)benzylamine |
| 9H- | purine, 6-[(phenylmethyl)amino]- |


