Articles:
hexanoic acid, 6-amino-
Notes:
an antifibrinolytic agent that acts by inhibiting plasminogen activators which have fibrinolytic properties. an antifibrinolytic agent that acts by inhibiting plasminogen activators which have fibrinolytic properties. Aminocaproic acid (marketed as Amicar) is a drug used to treat bleeding disorders. It is an antifibrinolytic agent that acts by inhibiting plasminogen activators which have fibrinolytic properties. It is a derivative of the amino acid lysine. It binds reversibly to the kringle domain of plasminogen and blocks the binding of plasminogen to fibrin and its activation to plasmin. [HMDB]
| CAS Number: | 60-32-2 | 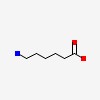 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 87867-96-7 | |
| ECHA EINECS - REACH Pre-Reg: | 200-469-3 | |
| FDA UNII: | U6F3787206 | |
| Nikkaji Web: | J1.398F | |
| Beilstein Number: | 0906872 | |
| MDL: | MFCD00008238 | |
| XlogP3: | -3.00 (est) | |
| Molecular Weight: | 130.16704000 | |
| Formula: | C6 H12 N O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: pharmaceuticals / chemical synthisis
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white crystalline powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 205.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 255.64 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.005000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 227.00 °F. TCC ( 108.40 °C. ) (est) |
| logP (o/w): | 0.027 (est) |
| Soluble in: | |
| water, 2.56e+005 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
not used anymore |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Aminocaproic acid >98%
Odor: characteristic Use: Aminocaproic acid is a derivative and analogue of the amino acid lysine, which makes it an effective inhibitor for enzymes that bind that particular residue. Such enzymes include proteolytic enzymes like plasmin, the enzyme responsible for fibrinolysis. |
| Glentham Life Sciences |
| 6-Aminohexanoic acid, USP grade |
| Glentham Life Sciences |
| 6-Aminohexanoic acid |
| Indis NV |
| For experimental / research use only. |
| 6-Aminocaproic Acid |
| Penta International |
| AMINOCAPROIC ACID USP |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| 6-Aminocaproic Acid ≥99% (titration), powder |
| TCI AMERICA |
| For experimental / research use only. |
| 6-Aminocaproic Acid >98.0%(T) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-dog LD50 > 7000 mg/kg Drugs in Japan Vol. 6, Pg. 79, 1982. intravenous-dog LDLo 2150 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Pharmacologist. Vol. 3, Pg. 62, 1961. intravenous-guinea pig LDLo 19800 mg/kg Anesthesie, Analgesie, Reanimation. Vol. 22, Pg. 481, 1965. oral-man TDLo 1778 mg/kg/8D- KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: HEMATURIA American Journal of Kidney Diseases. Vol. 8, Pg. 441, 1986. intravenous-mouse LD50 4900 mg/kg BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: DYSPNEA Anesthesie, Analgesie, Reanimation. Vol. 22, Pg. 481, 1965. oral-mouse LD50 14300 mg/kg Drugs in Japan Vol. 6, Pg. 79, 1982. intravenous-rabbit LD50 2000 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 9, Pg. 759, 1967. oral-rat LD > 10000 mg/kg Pharmacologist. Vol. 3, Pg. 62, 1961. intraperitoneal-rat LD50 7000 mg/kg Pharmacologist. Vol. 3, Pg. 62, 1961. intravenous-rat LD50 3300 mg/kg Pharmacologist. Vol. 3, Pg. 62, 1961. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 5790 mg/kg Drugs in Japan Vol. -, Pg. 62, 1995. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | pharmaceuticals / chemical synthisis | ||
| Recommendation for 6-aminocaproic acid usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for 6-aminocaproic acid flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 60-32-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 564 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 6-aminohexanoic acid | |
| Chemidplus: | 0000060322 |
| RTECS: | MO6300000 for cas# 60-32-2 |
References:
| 6-aminohexanoic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 60-32-2 |
| Pubchem (cid): | 564 |
| Pubchem (sid): | 134972930 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| Golm Metabolome Database: | Search |
| Metabolomics Database: | Search |
| UM BBD: | Search |
| KEGG (GenomeNet): | C02378 |
| HMDB (The Human Metabolome Database): | HMDB01901 |
| FooDB: | FDB022729 |
| Export Tariff Code: | 2922.49.4050 |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| acepramin | |
| acikaprin | |
| afibrin | |
| amikar | |
| 6- | amino hexanoic acid |
| 6- | amino-hexanoic acid |
| 6- | amino-N-caproic acid |
| 6- | amino-N-hexanoic acid |
| 6- | aminocaproicacid |
| 6- | aminohexanoic acid |
| 6- | aminohexanoicacid |
| capranol | |
| caproamin | |
| epsamon | |
| hemopar | |
| hexanoic acid, 6-amino- | |
| respramin |



