Articles:
1,4-naphthalenedione, 2-amino-3-chloro-
Notes:
induces cell differentiation in hl-60 cells.
| CAS Number: | 2797-51-5 | 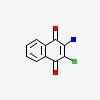 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 220-529-2 | |
| FDA UNII: | JN6NK7K14F | |
| Nikkaji Web: | J3.040F | |
| Beilstein Number: | 2094762 | |
| MDL: | MFCD00001680 | |
| XlogP3: | 2.10 (est) | |
| Molecular Weight: | 207.61522000 | |
| Formula: | C10 H6 Cl N O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: herbicides / pesticides
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 199.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 310.23 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.001000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 287.00 °F. TCC ( 141.40 °C. ) (est) |
| logP (o/w): | 2.120 |
| Soluble in: | |
| water, 6300 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| 2-Amino-3-chloro-1,4-naphthoquinone |
| Sigma-Aldrich |
| For experimental / research use only. |
| Quinoclamine PESTANAL®, analytical standard |
| TCI AMERICA |
| For experimental / research use only. |
| 2-Amino-3-chloro-1,4-naphthoquinone >98.0%(HPLC)(N) |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-mouse LD50 800 mg/kg Journal of Medicinal Chemistry. Vol. 26, Pg. 570, 1983. intravenous-mouse LD50 320 mg/kg U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#03360 oral-mouse LD50 1260 mg/kg Farm Chemicals Handbook. Vol. -, Pg. C208, 1991. intraperitoneal-rat LD50 315 mg/kg Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 18, Pg. S19, 1993. oral-rat LD50 1360 mg/kg Farm Chemicals Handbook. Vol. -, Pg. C208, 1991. | |
| Dermal Toxicity: | |
|
skin-mouse LD50 > 2000 mg/kg Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 18, Pg. S19, 1993. skin-rat LD50 > 5000 mg/kg Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 18, Pg. S19, 1993. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 > 790 mg/m3/4H Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 18, Pg. S19, 1993. | |
Safety in Use Information:
| Category: | herbicides / pesticides | ||
| Recommendation for quinoclamine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for quinoclamine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Reasoned opinion on the review of the existing maximum residue levels (MRLs) for quinoclamine according to Article 12 of Regulation (EC) No 396/2005 View page or View pdf | |
| Retrospective analysis of the immunotoxic effects of plant protection products as reported in the Draft Assessment Reports for their peer review at EU level View page or View pdf | |
| EPI System: | View |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 17748 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 2-amino-3-chloronaphthalene-1,4-dione | |
| Chemidplus: | 0002797515 |
| RTECS: | QL7350000 for cas# 2797-51-5 |
References:
| 2-amino-3-chloronaphthalene-1,4-dione | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 17748 |
| Pubchem (sid): | 134983089 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C18584 |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2922.39.4500 |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| ACN | |
| 3- | amino-2-chloronaphthalene-1,4-dione |
| 2- | amino-3-chlor-1,4-naphthochinone |
| 2- | amino-3-chloro-[1,4]naphthoquinone |
| 2- | amino-3-chloro-1,4-naphthoquinone |
| 2- | amino-3-chloro-1,4-naphtoquinone |
| 2- | amino-3-chloronaphthalene-1,4-dione |
| 2- | amino-3-chloronaphthoquinone |
| 2- | chloro-3-amino-1,4-naphthoquinone |
| 1,4- | naphthalenedione, 2-amino-3-chloro- |

