Articles:
ethane, 1,1,2-trichloro-1,2,2-trifluoro-
Notes:
Used in freezing of foods
CFC-113 is a very unreactive chlorofluorocarbon, that will stay in the atmosphere for a great deal of time if it is released. CFC-113 will stay in the atmosphere long enough that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation, creating chlorine radicals, which can in turn react with ozone molecules to form molecular oxygen (O2), leading to the overall depletion of stratospheric ozone. The amount of CFC-113 in the atmosphere has stayed relatively stable, at about 80 parts per trillion, since the early 1990s.; CFC-113 was one of the many forms of CFCs that were made to eliminate toxic and flammable substances in the areas that they were used. It has been used as a cooling agent in refrigerants and air conditioners, aerosol propellant, and a cleansing agent for electrical and electronic components. CFC-113 is one of the three most popular CFCs, along with CFC-11 and CFC-12 and saw much use in its time. CFC-113 has a unique property that makes it perfect for cooling systems. When it is in a gas form and compressed, it heats up, when it is expanded, it cools. This makes them ideal for the vapor compression cycle systems. They were also very desirable because of their low toxicity, non-flammability, thermophysical properties, and normal boiling point. CFC-113 also has a flexible form so it was used in the production of plastics, packaging material, insulation, foams for cushioning, and things like the soles of your shoes. CFC-113 has such a low flammability and low toxicity that it was also used as a cleaner for delicate electrical equipment, fabrics, and even metals. Because it would not warm the product it was cleaning, catch fire with a spark or react to and other chemicals it was ideal for this purpose. CFC-113 in laboratory analytics has been replaced by other solvents.; Trichlorotrifluoroethane, also called 1,1,2-Trichloro-1,2,2-trifluoroethane or CFC-113 is a chlorofluorocarbon. It has the formula Cl2FC-CClF2.
| CAS Number: | 76-13-1 | 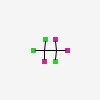 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 200-936-1 | |
| FDA UNII: | 0739N04X3A | |
| Nikkaji Web: | J1.947J | |
| Beilstein Number: | 1740335 | |
| MDL: | MFCD00000782 | |
| XlogP3: | 3.20 (est) | |
| Molecular Weight: | 187.37320960 | |
| Formula: | C2 Cl3 F3 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: extraction solvents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Additive: | 1,1,2-Trichlorotrifluoroethane |
| FDA Mainterm (SATF): | 76-13-1 ; CHLOROFLUOROCARBON 113 |
| FDA Regulation: | |
| FDA PART 173 -- SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION Subpart D--Specific Usage Additives Sec. 173.342 Chlorofluorocarbon 113 and perfluorohexane. | |
Physical Properties:
| Appearance: | colorless clear liquid (est) |
| Assay: | 99.80 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 1.57000 @ 25.00 °C. |
| Refractive Index: | 1.35800 @ 20.00 °C. |
| Melting Point: | -35.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 47.00 to 48.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 363.000000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 2.00 °F. TCC ( -16.80 °C. ) (est) |
| logP (o/w): | 3.160 |
| Soluble in: | |
| water, 170 mg/L @ 25 °C (exp) | |
| alcohol | |
| chloroform | |
| ether | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| 1,1,2-Trichlorotrifluoroethane HPLC grade, ≥99.9% |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| R 02 - Risk of explosion by shock, friction, fire or other sources of ignition. | |
|
R 52/53 - Harmful to qauatic organisms, may cause long-term adverse effects in the aquatic environment. R 59 - Dangerous for the ozone layer. S 02 - Keep out of the reach of children. S 59 - Refer to manufacturer for information on recovery/recycling. S 61 - Avoid release to the environment. Refer to special instructions/safety data sheet. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 43000 mg/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES SKIN AND APPENDAGES (SKIN): HAIR: OTHER Journal of Medicinal Chemistry. Vol. 7, Pg. 378, 1964. oral-guinea pig LDLo > 10000 mg/kg National Technical Information Service. Vol. OTS0520705 intravenous-mouse LD50 9000 mg/kg AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) National Technical Information Service. Vol. OTS0520355 unreported-mouse LD50 40000 mg/kg United States Patent Document. Vol. #4164653 oral-rabbit LDLo 17000 mg/kg American Industrial Hygiene Association Journal. Vol. 29, Pg. 521, 1968. | |
| Dermal Toxicity: | |
|
skin-rabbit LD50 > 11000 mg/kg American Industrial Hygiene Association Journal. Vol. 29, Pg. 521, 1968. | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 38500 ppm/4H BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: EXCITEMENT BEHAVIORAL: ATAXIA National Technical Information Service. Vol. OTS0520341 inhalation-guinea pig LC50 > 12 pph/2H Archives des Maladies Professionnelles de Medecine du Travail et de Securite Sociale. Vol. 29, Pg. 381, 1968. inhalation-mouse LC50 260000 mg/m3/2H LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: ATAXIA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Trudy Leningradskogo Sanitarno-Gigienicheskogo Meditsinskogo Instituta. Vol. 75, Pg. 241, 1963. inhalation-rabbit LC50 59500 ppm/2H LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION BEHAVIORAL: EXCITEMENT SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE National Technical Information Service. Vol. OTS0520343 | |
Safety in Use Information:
| Category: | extraction solvents | ||
| Recommendation for 1,1,2-trichlorotrifluoroethane usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for 1,1,2-trichlorotrifluoroethane flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| EPA-Iris: | IRIS |
| NIOSH International Chemical Safety Cards: | search |
| NIOSH Pocket Guide: | search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| Carcinogenic Potency Database: | Search |
| EPA Substance Registry Services (TSCA): | 76-13-1 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6428 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 3082 |
| WGK Germany: | 2 |
| 1,1,2-trichloro-1,2,2-trifluoroethane | |
| Chemidplus: | 0000076131 |
| EPA/NOAA CAMEO: | hazardous materials |
| RTECS: | 76-13-1 |
References:
| 1,1,2-trichloro-1,2,2-trifluoroethane | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 76-13-1 |
| Pubchem (cid): | 6428 |
| Pubchem (sid): | 134971388 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | HMDB31155 |
| FooDB: | FDB003169 |
| Export Tariff Code: | 2903.40.0020 |
| FDA Listing of Food Additive Status: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: grades: technical; spectrophotometric. | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| arcton 113 | |
| asahifron 113 | |
| chlorofluorocarbon 113 | |
| daiflon 113 | |
| delifrene 113 | |
| ethane, 1,1,2-trichloro-1,2,2-trifluoro- | |
| flugene 113 | |
| forane 113 | |
| freon F113 | |
| frigen 113 | |
| fronsolve 113 | |
| genetron 113 | |
| halocarbon 113 | |
| isceon 113 | |
| khladon 113 | |
| refrigerant 113 | |
| 1,1,2- | trichloro-1,2,2-trifluorethane |
| trichloro-1,2,2-trifluoroethane | |
| 1,1,2- | trichloro-1,2,2-trifluoroethane |
| 1,2,2- | trichlorotrifluoroethane |
| 1,1,2- | trifluoro-1,2,2-trichloroethane |
| 1,1,2- | trifluorotrichloroethane |