Articles:
berbaman, 6,6',7,12-tetramethoxy-2,2'-dimethyl-, (1b)- (9CI)
Notes:
a bisbenzylisoquinoline; merck reference is for 1-tetrandrine; exhibits antifibrogenic activity.
| CAS Number: | 518-34-3 | 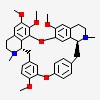 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 5990-67-0 | |
| FDA UNII: | 29EX23D5AJ | |
| Nikkaji Web: | J130.138A | |
| Beilstein Number: | 0877811 | |
| MDL: | MFCD08689909 | |
| XlogP3-AA: | 6.40 (est) | |
| Molecular Weight: | 622.76194000 | |
| Formula: | C38 H42 N2 O6 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: cosmetic ingredient for skin conditioning
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 217.50 °C. @ 760.00 mm Hg |
| Boiling Point: | 710.50 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 348.00 °F. TCC ( 175.80 °C. ) (est) |
| logP (o/w): | 3.550 (est) |
| Soluble in: | |
| water, 1.356e-005 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Tetrandrine 98% |
| BOC Sciences |
| For experimental / research use only. |
| Tetrandrine 98%
Odor: characteristic Use: Tetrandrine is a bisbenylisoquinoline alkaloid isolated from the dried root of Stephenia tetrandra S Moore. Tetrandrine exhibits very broad pharmacological actions, including anti-tumor activity. |
| Coompo |
| For experimental / research use only. |
| Tetrandrine from Plants ≥98%
Odor: characteristic Use: Tetrandrine has anti-inflammatory, immunologic and antiallergenic effects. It inhibits the degranulation of mast cells. It has a "Quinidine like" anti-arrhythmic effect. It has vasodilatory properties and can therefore reduce blood pressure. Tetrandrine has potential therapeutic value to prevent excess scarring / fibrosis in conjunctiva following trabeculectomy or in patients with severe conjunctival inflammation. Tetrandrine has anti-inflammatory and anti-fibrogenic actions, which make tetrandrine and related compounds potentially useful in the treatment of lung silicosis, liver cirrhosis, and rheumatoid arthritis.
Tetrandrine may have potential use for the treatment of liver disease and liver cancer. and it also has anti-tumor/growth activities. However, the signaling pathways of tetrandrine-induced growth arrest and apoptosis in cancer cells remain unclear. We investigated the molecular mechanisms of tetrandrine-induced apoptosis and growth arrest in human lung carcinoma cells. Upon treatment with tetrandrine, a time-dependent inhibition of cell growth was observed and cells developed many of the hallmark features of apoptosis. Flow cytometry analysis confirmed that tetrandrine increased populations of both apoptotic sub-G1 and G1 phase. Tetrandrine-induced growth inhibition was associated with induction of Cdk inhibitor p21, inhibition of cyclin D1 and activation of caspase-3. Tetrandrine also affected the expression patterns of cytoskeletons including distribution of F-actin and expression level of microtubule. These results suggest that tetrandrine merits further investigation as a cell cycle blocker as well as a cancer chemopreventive agent.
Tetrandrine presents antiallergic effects, inhibitory effects on pulmonary vessels and airway smooth muscle contraction, and platelet aggregation via its nonspecific calcium channel antagonism that suggested its potential in the treatment of asthma, pulmonary hypertension and chronic obstructive pulmonary disease (COPD). In general, the clinical results to date with tetrandrine in asthma and pulmonary hypertension have been exciting. |
| ExtraSynthese |
| For experimental / research use only. |
| (S,S)-(+)-Tetrandrine |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Tetrandrine ≥98% |
| Sigma-Aldrich |
| For experimental / research use only. |
| Tetrandrine
analytical standard, for drug analysis |
| TCI AMERICA |
| For experimental / research use only. |
| Tetrandrine >98.0%(HPLC)(T) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-cat LDLo 40 mg/kg CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: TREMOR Zhongcaoyao. Chinese Traditional and Herbal Medicine. Vol. 25, Pg. 610, 1994. intraperitoneal-mouse LD50 41300 ug/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD "Zhongliu Yanjiu" Cancer Review, Yu, R., et al., eds., Shanghai Science/Technology Publisher,Peop. Rep. China, 1994Vol. -, Pg. 216, 1994. intravenous-mouse LD50 37500 ug/kg Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 25, Pg. 39, 1990. intravenous-rabbit LDLo 15 mg/kg LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES CARDIAC: OTHER CHANGES BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 25, Pg. 39, 1990. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic ingredient for skin conditioning | ||
| Recommendation for tetrandrine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for tetrandrine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 73078 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| Chemidplus: | 0000518343 |
| RTECS: | XE9350000 for cas# 518-34-3 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 73078 |
| Pubchem (sid): | 135030415 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C09654 |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2939.80.0000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| found in nature |
Synonyms:
| berbaman, 6,6',7,12-tetramethoxy-2,2'-dimethyl-, (1b)- (9CI) | |
| fanchinine | |
| hanfangchin A | |
| sinomenine A | |
| (1b)-6,6,7,12- | tetramethoxy-2,2?-dimethylberbaman |
| (1b)-6,6',7,12- | tetramethoxy-2,2'-dimethylberbaman |
| tetrandrin | |
| (+)- | tetrandrine |
| (S,S)- | tetrandrine |
| (S,S)-(+)- | tetrandrine |

