Articles:
ethanone, 2-((2R,6S)-6-((2S)-2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenyl-, hydrochloride
Notes:
an alkaloid that has actions similar to nicotine on nicotinic cholinergic receptors but is less potent. it has been proposed for a variety of therapeutic uses including in respiratory disorders, peripheral vascular disorders, insomnia, and smoking cessation.
| CAS Number: | 134-63-4 | 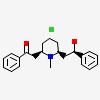 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 205-150-2 | |
| FDA UNII: | 96J834CB88 | |
| Beilstein Number: | 3920196 | |
| MDL: | MFCD00082455 | |
| Molecular Weight: | 373.92256000 | |
| Formula: | C22 H28 Cl N O2 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: information only not used for fragrances or flavors
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 510.50 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 505.00 °F. TCC ( 262.50 °C. ) (est) |
| logP (o/w): | 3.610 (est) |
| Soluble in: | |
| water, 659.5 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Lobeline hydrochloride 98% |
| BOC Sciences |
| For experimental / research use only. |
| a-Lobeline Hydrochcloride > 95%
Odor: characteristic Use: a-Lobeline Hydrochcloride is a neuronal nicotinic acetylcholine receptor agonist. It is also a CNS stimulant. |
| ExtraSynthese |
| For experimental / research use only. |
| alpha-Lobeline hydrochloride |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Lobeline Hydrochloride ≥97% |
| Sigma-Aldrich |
| For experimental / research use only. |
| Lobeline Hydrochloride European Pharmacopoeia (EP) Reference Standard |
| TCI AMERICA |
| For experimental / research use only. |
| Lobeline Hydrochloride >98.0%(HPLC)(T) |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
parenteral-frog LDLo 150 mg/kg PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 163, Pg. 279, 1931. intraperitoneal-mouse LD50 39900 ug/kg Drugs in Japan Vol. 6, Pg. 913, 1982. intravenous-mouse LD50 7800 ug/kg Drugs in Japan Vol. 6, Pg. 913, 1982. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 87500 ug/kg Drugs in Japan Vol. 6, Pg. 913, 1982. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | information only not used for fragrances or flavors | ||
| Recommendation for lobeline hydrochloride usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for lobeline hydrochloride flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 17751021 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| 2-[(2R,6S)-6-[(2R)-2-hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl]-1-phenylethanone;hydrochloride | |
| Chemidplus: | 0000134634 |
| RTECS: | OJ8490100 for cas# 134-63-4 |
References:
| 2-[(2R,6S)-6-[(2R)-2-hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl]-1-phenylethanone;hydrochloride | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 17751021 |
| Pubchem (sid): | 135059924 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2933.39.9100 |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| acetophenone, 2-(6-(beta-hydroxyphenethyl)-1-methyl-2-piperidyl)-, hydrochloride, (-)- | |
| ethanone, 2-((2R,6S)-6-((2S)-2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenyl-, hydrochloride | |
| 2-[(2R,6S)-6-[(2R)-2- | hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl]-1-phenylethanone;hydrochloride |
| 2-{(2R,6S)-6-[(2R)-2- | hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl}-1-phenylethanone hydrochloride (1:1) |
| lobeline HCl | |
| (-)- | lobeline hydrochloride |
| alpha- | lobeline hydrochloride |

