Articles:
(1R,9S)-7,11-diazatricyclo[7.3.1.02,7]trideca-2,4-dien-6-on
Notes:
None found
| CAS Number: | 485-35-8 | 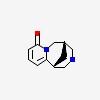 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 3728-36-7 | |
| ECHA EINECS - REACH Pre-Reg: | 207-616-0 | |
| FDA UNII: | 53S5U404NU | |
| Nikkaji Web: | J9.571K | |
| Beilstein Number: | 0083882 | |
| MDL: | MFCD00136048 | |
| XlogP3-AA: | 0.20 (est) | |
| Molecular Weight: | 190.24578000 | |
| Formula: | C11 H14 N2 O | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 152.50 °C. @ 760.00 mm Hg |
| Boiling Point: | 413.04 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 398.00 °F. TCC ( 203.60 °C. ) (est) |
| logP (o/w): | 0.172 (est) |
| Soluble in: | |
| water, 1.511e+004 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Cytisine 98% |
| BOC Sciences |
| For experimental / research use only. |
| Cytisine >98%
Odor: characteristic Use: Cytisine is a nicotinic acetylcholine receptor agonist.It has been used medically to help with smoking cessation. |
| Coompo |
| For experimental / research use only. |
| Cytisine from Plants ≥98%
Odor: characteristic Use: Cytisine is an acetylcholine agonist, and has strong binding affinity for the nicotinic acetylcholine receptor. As a pharmaceutical preparation, it is available for the treatment of tobacco smoking. It has been available in former socialist economy (FSE) countries for more than 40 years as an aid to smoking cessation under the brand name Tabex produced by the Bulgarian pharmaceutical company Sopharma AD. The synthetic drug varenicline, which has some structural and pharmacological similarities to cytisine, was approved in 2006 as a smoking cessation drug.
In 2011, a randomized controlled trial with 740 patients found cytisine improved 12-month abstinence from nicotine from 2.4% with placebo to 8.4% with cytisine. |
| ExtraSynthese |
| For experimental / research use only. |
| Cytisine |
| Glentham Life Sciences |
| Cytisine |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Cytisine ≥99% |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Cytisine ≥99%, powder |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-cat LD50 400 ug/kg BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) Izvestiya Akademii Nauk Tadzhikskoi SSR, Otdelenie Biologicheskikh Nauk. Proceedings of the Academy of Sciences of the Tadzhik SSR, Department of Biological Sciences. Vol. (2), Pg. 104, 1978. intravenous-dog LDLo 16 mg/kg "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 589, 1948. intraperitoneal-mouse LD50 8550 ug/kg Zhongcaoyao. Chinese Traditional and Herbal Medicine. Vol. 18, Pg. 214, 1987. intravenous-mouse LD50 1730 ug/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: REGIDITY British Journal of Pharmacology. Vol. 35, Pg. 161, 1969. oral-mouse LD50 101 mg/kg BEHAVIORAL: REGIDITY BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD British Journal of Pharmacology. Vol. 35, Pg. 161, 1969. | |
| Dermal Toxicity: | |
|
subcutaneous-guinea pig LDLo 40 mg/kg "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 589, 1948. subcutaneous-mouse LD50 11764 ug/kg Farmakologiya i Toksikologiya Vol. 4(1), Pg. 34, 1941. subcutaneous-rabbit LD50 5 mg/kg Farmakologiya i Toksikologiya Vol. 4(1), Pg. 34, 1941. subcutaneous-rat LD50 8750 ug/kg Farmakologiya i Toksikologiya Vol. 4(1), Pg. 34, 1941. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for cytisine usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for cytisine flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 485-35-8 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 10235 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| Chemidplus: | 0000485358 |
| RTECS: | HA4025000 for cas# 485-35-8 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 10235 |
| Pubchem (sid): | 134975346 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| HMDB (The Human Metabolome Database): | Search |
| Export Tariff Code: | 2933.90.9500 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| genet Search Trop Picture |
Synonyms:
| baptitoxin | |
| baptitoxine | |
| cytiton | |
| cytitone | |
| (1R,9S)-7,11- | diazatricyclo[7.3.1.02,7]trideca-2,4-dien-6-on |
| (1R,9S)-7,11- | diazatricyclo[7.3.1.02,7]trideca-2,4-dien-6-one |
| (1R-5S)-1,2,3,4,5,6- | hexahydro-1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one |
| (1R,5S)-1,2,3,4,5,6- | hexahydro-1,5-methano-8H-pyrido[1,2-a][1,5]diazocin-8-one |
| (1R,5S)-1,2,3,4,5,6- | hexahydro-1,5-methano-pyrido[1,2-a][1,5]diazocin-8-one |
| (1R,5S)-1,2,3,4,5,6- | hexahydro-8H-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one |
| laburnin | |
| 1,5- | methano-8H-pyrido(1,2-a)(1,5)diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1R,5S)- |
| 1,5- | methano-8H-pyrido[1,2-a][1,5]diazocin-8-one, 1,2,3,4,5,6-hexahydro-, (1R,5S)- |
| tabax | |
| (1R,5S)-3,4,5,6- | tetrahydro-1H-1,5-methanopyrido[1,2-a][1,5]diazocin-8(2H)-one |
| tsitafat | |
| tsitizin | |
| ulexin | |
| ulexine |

