Articles:
oxirane, methyl, polymer with oxirane (98 mol EO, 67 mol PO average molar ratio)
Notes:
a copolymer of polyethylene and polypropylene ether glycol. it is a non-ionic polyol surface-active agent used medically as a fecal softener and in cattle for prevention of bloat.
| CAS Number: | 9003-11-6 | 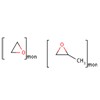 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 106717-66-2 | |
| Formula: | (C3 H6 O.C2 H4 O)x- | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: emulsifiers and foaming agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 275.31 °C. @ 760.00 mm Hg (est) |
| Vapor Pressure: | 0.001000 mmHg @ 25.00 °C. (est) |
| Flash Point: | 249.00 °F. TCC ( 120.30 °C. ) (est) |
| logP (o/w): | -1.293 (est) |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
surfactants surfactant - emulsifying |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Poloxamer Liquid 95% |
| Penta International |
| POLOXAMER 407 FCC |
| Penta International |
| POLOXAMER 407 NF |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-mouse LD50 175 mg/kg Microvascular Research. Vol. 8, Pg. 320, 1974. oral-mouse LD50 3000 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(12), Pg. 56, 1984. oral-mouse LD50 > 15000 mg/kg Drug and Chemical Toxicology. Vol. 1, Pg. 89, 1977/1978. oral-mouse LD50 45000 mg/kg Toxicology and Applied Pharmacology. Vol. 16, Pg. 675, 1970. intraperitoneal-mouse LDLo 5000 mg/kg Therapie. Vol. 14, Pg. 721, 1959. intravenous-mouse LDLo 6000 mg/kg Therapie. Vol. 14, Pg. 721, 1959. oral-mouse LDLo 15000 mg/kg Therapie. Vol. 14, Pg. 721, 1959. oral-rabbit LD50 35000 mg/kg Toxicology and Applied Pharmacology. Vol. 16, Pg. 675, 1970. oral-rat LD50 5700 mg/kg GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(12), Pg. 56, 1984. intraperitoneal-rat LDLo 10000 mg/kg Therapie. Vol. 14, Pg. 721, 1959. oral-rat LDLo 16000 mg/kg Toxicology and Applied Pharmacology. Vol. 16, Pg. 675, 1970. | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
|
inhalation-rat LC50 320 mg/m3/4H LIVER: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES National Technical Information Service. Vol. OTS0539401 | |
Safety in Use Information:
| Category: | emulsifiers and foaming agents | ||
| Recommendation for peg/ppg-98/67 copolymer usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for peg/ppg-98/67 copolymer flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to an 11th list of substances for food contact materials View page or View pdf | |
| Review of substances/agents that have direct beneficial effect on the environment: mode of action and assessment of efficacy View page or View pdf | |
| EPI System: | View |
| Daily Med: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 9003-11-6 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 24751 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| 2-methyloxirane; oxirane (98;67) | |
| Chemidplus: | 0009003116 |
References:
| 2-methyloxirane; oxirane (98;67) | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 9003-11-6 |
| Pubchem (cid): | 24751 |
| Pubchem (sid): | 134991398 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| FDA Indirect Additives used in Food Contact Substances: | View |
| HMDB (The Human Metabolome Database): | Search |
| FDA Listing of Food Additive Status: | View |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •trade names: bloat guard; therabloat •a block polymer of ethylene oxide and propylene oxide. | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| emollients | ||
| emulsifying agents | ||
| humectants | ||
| solvents | ||
| surfactants |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| methyl oxirane polymer with oxirane (98;67) | |
| 2- | methyloxirane; oxirane (98;67) |
| oxirane, methyl, polymer with oxirane (98 mol EO, 67 mol PO average molar ratio) | |
| pluracare F 127 | |
| pluronic P-123 | |
| poloxamer 407 |

