Articles:
hyaluronic acid, sodium salt
Notes:
a natural high-viscosity mucopolysaccharide with alternating beta (1-3) glucuronide and beta (1-4) glucosaminidic bonds. it is found in the umbilical cord, in vitreous humor, in synovial fluid, in pathologic joints, in group a and c hemolytic streptococci, and in wharton's jelly. a high urinary level is found in progeria.
| CAS Number: | 9067-32-7 | 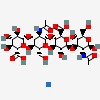 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 34448-35-6 | |
| FDA UNII: | YSE9PPT4TH | |
| MDL: | MFCD01773053 | |
| Molecular Weight: | 799.64404928 | |
| Formula: | C28 H44 N2 Na O23 + | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: dietary supplements: EFSA: cannot be assessed.
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 1274.00 to 1275.00 °C. @ 760.00 mm Hg (est) |
| Flash Point: | 1336.00 °F. TCC ( 724.50 °C. ) (est) |
| logP (o/w): | -6.623 (est) |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
humectants skin conditioning |
Suppliers:
| AIDP |
| Hyaluronic Acid Sodium 99% HA 99% min. |
| American International Chemical, LLC. |
| Sodium Hyaluronate |
| AuNutra® Industries |
| Hyaluronic Acid Sodium |
| BOC Sciences |
| For experimental / research use only. |
| Hyaluronic acid sodium
Odor: characteristic Use: Hyaluronic Acid, Sodium Salt is sodium salt form of Hyaluronic Acid. Hyaluronic acid, a mucopolysaccharide with high-viscosity, traditionally extracted from rooster combs, but now it is mainly produced via streptococcal fermentation. |
| Charkit Chemical |
| SODIUM HYALURONATE (HYALURONIC ACID) |
| Charkit Chemical |
| SODIUM HYALURONATE 1% SOLUTION |
| Charkit Chemical |
| SODIUM HYALURONATE POWDER |
| Chemical-navi - Nikkol |
| SODIUM HYALURONATE POWDER (CHA) |
| Contipro |
| Hyaluronan Oligosaccharides
Odor: characteristic Use: Sodium hyaluronate oligosaccharides (hyaluronan oligomers) are non-sulphated glycosaminoglycans. The structure is given by an enzyme used for the digestion that cleaves selectively β (1?4) glycosidic bonds between disaccharide units of sodium hyaluronate. The enzymatic digestion is followed by chromatographic separation when the pure, size-uniformed species are obtained. |
| Contipro |
| Sodium hyaluronate LMW
Odor: characteristic Use: Sodium hyaluronate (hyaluronan, sodium salt of hyaluronic acid) is a non-sulphated glycosaminoglycan, a naturally occuring polysaccharide. |
| Contipro |
| Sodium Hyaluronate
Odor: characteristic Use: Sodium hyaluronate is a non-sulphated glycosaminoglycan, a naturally occuring polysaccharide. |
| ECSA Chemicals |
| SODIUM HYALURONATE 0.2-0.4MDa |
| ECSA TRADE THE MOST UPDATED FINANCIAL PUBLICATION ON THE WORLD OF CHEMISTRY |
| ECSA Chemicals |
| SODIUM HYALURONATE 1.0-1.4 Mda |
| ECSA Chemicals |
| SODIUM HYALURONATE 1.00MDA - 1.80MDA |
| Glentham Life Sciences |
| Hyaluronic acid sodium salt, m.w. 1.0 - 1.5 MDa |
| Glentham Life Sciences |
| Hyaluronic acid sodium salt, m.w. 3,000 - 5,000 |
| Glentham Life Sciences |
| Hyaluronic acid sodium salt, m.w. 30,000 - 50,000 |
| Glentham Life Sciences |
| Hyaluronic acid sodium salt, m.w. 50,000 - 100,000 |
| Jiangyin Healthway |
| Sodium Hyaluronate |
| New functional food ingredients |
| Jiangyin Healthway |
| Sodium Hyaluronate |
| Lipo Chemicals |
| Lipo® Hyaluronic Acid - 1% Solution
Odor: characteristic Use: Hyaluronic Acid (HA) is a naturally-occurring polyanionic polysaccharide. It is present in the intercellular domains of connective tissues and especially in the skin. Because of its unique rheological, viscoelastic and hygroscopic properties, HA plays a key role in protecting and stabilizing the skin at the cellular level. |
| Lipo Chemicals |
| Lipo® Hyaluronic Acid
Odor: characteristic Use: Hyaluronic Acid (HA) is a naturally-occurring polyanionic polysaccharide. It is present in the intercellular domains of connective tissues and especially in the skin. Because of its unique rheological, viscoelastic and hygroscopic properties, HA plays a key role in protecting and stabilizing the skin at the cellular level. |
| Lipo Chemicals |
| Orgasol® Hydra+
Odor: characteristic Use: Orgasol® Hydra+ brings immediate sensorial and visual effects combined with long-term efficacy. It offers a perfect velvet finish and an immediate optical smoothing effect to color cosmetics, turning daily makeup application into a moisturizing treatment. Orgasol® Hydra+ delivers hyaluronic acid to the skin. |
| Lipotec USA |
| Actiglide® |
| Lipotec USA |
| Actimoist® Bio 1 |
| Lipotec USA |
| Actimoist® Bio 2 |
| M.C.Biotec |
| Sodium Hyaluronate |
| Maypro Industries |
| Sodium Hyaluronate |
| Meafo |
| For experimental / research use only. |
| Sodium Hyaluronate 95% |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Hyaluronic Acid, Sodium Salt |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| Hyaluronic acid sodium salt |
| Solabia |
| Hyaluronic Acid BT
Odor: characteristic Use: Given its high molecular weight, Hyaluronic Acid BT maintains an optimum level of hydration. |
| Spec-Chem Industry |
| SpecKareTM HA (Sodium Hyaluronate) |
| TRI-K Industries |
| HyaClear® Solution
Odor: characteristic Use: A crystal clear solution of 1% sodium hyaluronate in water, HyaClear™ Solution helps skin retain moisture, decreases skin roughness, improves barrier function and decreases desquamation for smoother, healthier looking skin. It has a stable viscosity, clarity and pH at high formulating temperatures for easy formulation. |
| TRI-K Industries |
| Sodium Hyaluronate Powder
Odor: characteristic Use: A natural mucopolysaccharide produced by an all-natural fermentation and purification process. This natural humectant preserves skin’s elasticity and firmness. |
| United International |
| Sodium hyaluronate Nat. |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
oral-rat LD50 > 800 mg/kg Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. intraperitoneal-rat LD50 1770 mg/kg SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BLOOD: NORMOCYTIC ANEMIA Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. oral-rabbit LD50 > 1000 mg/kg Drugs in Japan Vol. -, Pg. 849, 1990. intraperitoneal-rabbit LD50 1820 mg/kg LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION BLOOD: NORMOCYTIC ANEMIA Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. oral-mouse LD50 > 2400 mg/kg Drugs in Japan Vol. -, Pg. 849, 1990. intraperitoneal-mouse LD50 1500 mg/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA SKIN AND APPENDAGES (SKIN): HAIR: OTHER Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 1013, 1984. | |
| Dermal Toxicity: | |
|
subcutaneous-rat LD50 > 4000 mg/kg BEHAVIORAL: ATAXIA Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. subcutaneous-rabbit LD50 > 2000 mg/kg SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. subcutaneous-mouse LD50 > 4000 mg/kg BEHAVIORAL: ATAXIA Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 12, Pg. 5369, 1984. subcutaneous-dog LD50 > 50 mg/kg GASTROINTESTINAL: NAUSEA OR VOMITING SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 19(Suppl | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | dietary supplements: EFSA: cannot be assessed. | ||
| Recommendation for sodium hyaluronate usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for sodium hyaluronate flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Inability to assess the safety of sodium hyaluronate added for nutritional purposes as a source of sodium in food supplements and the bioavailability of sodium from this source, based on the supporting dossier [1] View page or View pdf | |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 3084049 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| sodium;(2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carbo | |
| Chemidplus: | 0009067327 |
| RTECS: | MT7250000 for cas# 9067-32-7 |
References:
| sodium;(2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carbo | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 3084049 |
| Pubchem (sid): | 135232288 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| HMDB (The Human Metabolome Database): | Search |
| FDA Listing of Food Additive Status: | View |
| MedlinePlusSupp: | View |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| actiglide | |
| actimoist Bio 1 | |
| actimoist Bio 2 | |
| chlamyhyaluronic acid sodium salt | |
| chronosphere hyaluronic | |
| hyaluronate sodium | |
| hyaluronic acid sodium | |
| hyaluronic acid, sodium salt | |
| orgasol hydra+ | |
| sodium hyaluronate 1 solution macronan S | |
| sodium hyaluronate LMW | |
| sodium hyaluronate macronan PF | |
| sodium hyaluronate macronan-P | |
| sodium hyaluronate powder (CHA) |






