Articles:
1-(4-hydroxy-3-methoxyphenyl)decan-3-one
Notes:
induces apoptosis. From grains of paradise (Amomum melegueta) and ginger (Zingiber officinale)
Paradol is the active flavor constituent of the seeds of Guinea pepper (Aframomum melegueta). The seed is also known as Grains of paradise. Paradol has been found to have antioxidative and antitumor promoting effects. It is used in flavors as an essential oil to give spiciness. (Wikipedia)
| CAS Number: | 27113-22-0 | 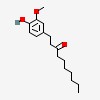 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 248-228-1 | |
| FDA UNII: | BO24ID7E9U | |
| Nikkaji Web: | J129.700G | |
| Beilstein Number: | 1984119 | |
| XlogP3: | 3.80 (est) | |
| Molecular Weight: | 278.39182000 | |
| Formula: | C17 H26 O3 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
| EFSA/JECFA Comments: | Register name to be changed to 1-(4-Hydroxy-3-methoxyphenyl)-3-decanone; CASrn: 27113-22-0 and EINECS: 248-228-1. | |
Category: cosmetic and flavor agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 2021 1-(4-hydroxy-3-methoxyphenyl)-decan-3-one |
| DG SANTE Food Flavourings: | 07.234 1-(4-hydroxy-3-methoxyphenyl)-decan-3-one |
| FEMA Number: | 4665 1-(4-hydroxy-3-methoxyphenyl)decan-3-one |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 27113-22-0 ; 1-(4-HYDROXY-3-METHOXYPHENYL)DECAN-3-ONE |
Physical Properties:
| Appearance: | white powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 31.00 to 33.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 406.00 to 408.00 °C. @ 760.00 mm Hg |
| Flash Point: | 284.00 °F. TCC ( 140.00 °C. ) |
| logP (o/w): | 4.225 (est) |
| Soluble in: | |
| alcohol | |
| water, 3.802 mg/L @ 25 °C (est) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Type: spicy | |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning |
Suppliers:
| BOC Sciences |
| For experimental / research use only. |
| Paradol >98% |
| Dalton Pharma Services |
| For experimental / research use only. |
| [6]-Paradol >95% |
| Jiangyin Healthway |
| 6-Paradol |
| New functional food ingredients |
| Parchem |
| 6-paradol |
| Symrise |
| SymRelief® S
Odor: characteristic Use: SymRelief® S is a synergistic blend of 2 nature identical skin soothing ingredients:
Dragosantol® 100 and [6]-paradol.
Dragosantol® 100 is the synthetic bisabolol with the highest purity available in the market today; [6]-paradol is one of the pungent principles of ginger and grains of paradise (Afromum melegueta), synthetic and nature-identical.
Each component synergistically contributes to SymRelief® S's broad skin soothing efficacy. SymRelief® S is perfect for sensitive skin as it significantly reduces redness and blotchiness already after 1 day of treatment. |
| Vigon International |
| SYMDECANOX HA |
Safety Information:
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-mouse LD50 270 mg/kg Journal of Toxicological Sciences. Vol. 16(Suppl.) | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | cosmetic and flavor agents | ||
| Recommendation for 6-paradol usage levels up to: | |||
| not for fragrance use. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 0.012 (μg/capita/day) | ||
| Modified Theoretical Added Maximum Daily Intake (mTAMDI): | 420 (μg/person/day) | ||
| Threshold of Concern: | 540 (μg/person/day) | ||
| Structure Class: | II | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 24 | |||
| Click here to view publication 24 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | - | |
| beverages(nonalcoholic): | 10.00000 | 50.00000 | |
| beverages(alcoholic): | 20.00000 | 100.00000 | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | 300.00000 | 800.00000 | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | 80.00000 | 250.00000 | |
| fish products: | - | - | |
| frozen dairy: | 50.00000 | 150.00000 | |
| fruit ices: | - | - | |
| gelatins / puddings: | 50.00000 | 150.00000 | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | 50.00000 | 150.00000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | 50.00000 | 150.00000 | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | 100.00000 | 250.00000 | |
| snack foods: | 20.00000 | 50.00000 | |
| soft candy: | 80.00000 | 200.00000 | |
| soups: | 20.00000 | 100.00000 | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
| Food categories according to Commission Regulation EC No. 1565/2000 (EC, 2000) in FGE.06 (EFSA, 2002a). According to the Industry the "normal" use is defined as the average of reported usages and "maximum use" is defined as the 95th percentile of reported usages (EFSA, 2002i). | |||
| Note: mg/kg = 0.001/1000 = 0.000001 = 1/1000000 = ppm. | |||
| average usage mg/kg | maximum usage mg/kg | ||
| Dairy products, excluding products of category 02.0 (01.0): | 0.50000 | 2.00000 | |
| Fats and oils, and fat emulsions (type water-in-oil) (02.0): | 0.20000 | 1.00000 | |
| Edible ices, including sherbet and sorbet (03.0): | 0.50000 | 2.50000 | |
| Processed fruit (04.1): | 0.40000 | 2.00000 | |
| Processed vegetables (incl. mushrooms & fungi, roots & tubers, pulses and legumes), and nuts & seeds (04.2): | - | - | |
| Confectionery (05.0): | 1.00000 | 5.00000 | |
| Chewing gum (05.0): | - | - | |
| Cereals and cereal products, incl. flours & starches from roots & tubers, pulses & legumes, excluding bakery (06.0): | 0.20000 | 1.00000 | |
| Bakery wares (07.0): | 2.00000 | 10.00000 | |
| Meat and meat products, including poultry and game (08.0): | 0.20000 | 1.00000 | |
| Fish and fish products, including molluscs, crustaceans and echinoderms (MCE) (09.0): | 0.20000 | 1.00000 | |
| Eggs and egg products (10.0): | - | - | |
| Sweeteners, including honey (11.0): | - | - | |
| Salts, spices, soups, sauces, salads, protein products, etc. (12.0): | 0.30000 | 1.50000 | |
| Foodstuffs intended for particular nutritional uses (13.0): | 0.50000 | 2.50000 | |
| Non-alcoholic ("soft") beverages, excl. dairy products (14.1): | 0.20000 | 1.00000 | |
| Alcoholic beverages, incl. alcohol-free and low-alcoholic counterparts (14.2): | 1.00000 | 5.00000 | |
| Ready-to-eat savouries (15.0): | 2.00000 | 10.00000 | |
| Composite foods (e.g. casseroles, meat pies, mincemeat) - foods that could not be placed in categories 01.0 - 15.0 (16.0): | 0.40000 | 2.00000 | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 22 (FGE.22): Ring-substituted phenolic substances from chemical groups 21 and 25 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) View page or View pdf | |
| Flavouring Group Evaluation 58 (FGE.58) Consideration of phenol derivatives evaluated by JECFA (55th meeting) structurally related to ring substituted phenolic substances evaluated by EFSA in FGE.22 (2006) (Commission Regulation (EC) No 1565/2000 of 18 July 2000) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 60 (FGE.60): Consideration of eugenol and related hydroxyallylbenzene derivatives evaluated by JECFA (65th meeting) structurally related to ring- substituted phenolic substances evaluated by EFSA in FGE.22 (2006) View page or View pdf | |
| Scientific Opinion on Flavouring Group Evaluation 22, Revision 1 (FGE.22Rev1): Ring-substituted phenolic substances from chemical groups 21 and 25 View page or View pdf | |
| EPI System: | View |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 94378 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| 1-(4-hydroxy-3-methoxyphenyl)decan-3-one | |
| Chemidplus: | 0027113220 |
References:
| 1-(4-hydroxy-3-methoxyphenyl)decan-3-one | |
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 94378 |
| Pubchem (sid): | 135049884 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C10482 |
| HMDB (The Human Metabolome Database): | HMDB30801 |
| FooDB: | FDB002743 |
| Typical G.C. | |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| FAO: | 1-(4-Hydroxy-3-methoxyphenyl)-decan-3-one |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| ginger rhizome PbMd Search Trop Picture | |
| ginger root oil CO2 extract PbMd Search Trop Picture | |
| ginger root oil CO2 extract australia @ 0.08% Data GC Search Trop Picture | |
| grains of paradise Search Trop Picture | |
| pepper melegueta pepper PbMd Search Trop Picture | |
| pepper sichuan pepper PbMd Search PMC Picture |
Synonyms:
| bisabolol, hydroxymethoxyphenyl decanone | |
| 3- | decanone, 1-(4-hydroxy-3-methoxyphenyl)- |
| 1-(4- | hydroxy-3-methoxyphenyl)-3-decanone |
| 1-(4- | hydroxy-3-methoxyphenyl)-decan-3-one |
| 1-(4- | hydroxy-3-methoxyphenyl)decan-3-one |
| [6]- | paradol |
| 5- | paradol |
| symdecanox HA (Vigon) | |
| symrelief S (Symrise) |



