Articles:
L-pteroylglutamic acid
Notes:
a member of the vitamin b family that stimulates the hematopoietic system. it is present in the liver and kidney and is found in mushrooms, spinach, yeast, green leaves, and grasses (poaceae). folic acid is used in the treatment and prevention of folate deficiencies and megaloblastic anemia. Dietary supplement
Folic acid (also known as vitamin B9, vitamin Bc or folacin) and folate (the naturally occurring form), as well as pteroyl-L-glutamic acid, pteroyl-L-glutamate, and pteroylmonoglutamic acid are forms of the water-soluble vitamin B9. [Wikipedia]
| CAS Number: | 59-30-3 | 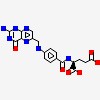 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 11096-55-2 | |
| ECHA EINECS - REACH Pre-Reg: | 200-419-0 | |
| FDA UNII: | 935E97BOY8 | |
| Nikkaji Web: | J1.392G | |
| Beilstein Number: | 100781 | |
| MDL: | MFCD00079305 | |
| XlogP3-AA: | -1.10 (est) | |
| Molecular Weight: | 441.40463000 | |
| Formula: | C19 H19 N7 O6 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: special dietary and nutritional additives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| FDA/DG SANTE Petitions, Reviews, Notices: | |
| FR DOC # 2012-14263 | Gruma Corporation, Spina Bifida Association, March of Dimes Foundation, American Academy of Pediatrics, Royal DSM N.V., and National Council of La Raza; Filing of Food Additive Petition View - petition PDF |
| FR DOC # 2012-17432 | Gruma Corporation, Spina Bifida Association, March of Dimes Foundation, American Academy of Pediatrics, Royal DSM N.V., and National Council of La Raza; Filing of Food Additive Petition; Correction View - petition PDF |
| FDA Mainterm (SATF): | 59-30-3 ; FOLIC ACID |
| FDA Regulation: | |
| FDA PART 101 -- FOOD LABELING Subpart E--Specific Requirements for Health Claims Sec. 101.79 Health claims: Folate and neural tube defects. FDA PART 101 -- FOOD LABELING Subpart A--General Provisions Sec. 101.9 Nutrition labeling of food. FDA PART 107 -- INFANT FORMULA Subpart D--Nutrient Requirements Sec. 107.100 Nutrient specifications. FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS Subpart B--Requirements for Specific Standardized Cereal Flours and Related Products Sec. 137.165 Enriched flour. FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS Subpart B--Requirements for Specific Standardized Cereal Flours and Related Products Sec. 137.185 Enriched self-rising flour. FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS Subpart B--Requirements for Specific Standardized Cereal Flours and Related Products Sec. 137.260 Enriched corn meals. FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS Subpart B--Requirements for Specific Standardized Cereal Flours and Related Products Sec. 137.305 Enriched farina. FDA PART 137 -- CEREAL FLOURS AND RELATED PRODUCTS Subpart B--Requirements for Specific Standardized Cereal Flours and Related Products Sec. 137.350 Enriched rice. FDA PART 139 -- MACARONI AND NOODLE PRODUCTS Subpart B--Requirements for Specific Standardized Macaroni and Noodle Products Sec. 139.115 Enriched macaroni products. FDA PART 139 -- MACARONI AND NOODLE PRODUCTS Subpart B--Requirements for Specific Standardized Macaroni and Noodle Products Sec. 139.122 Enriched nonfat milk macaroni products. FDA PART 139 -- MACARONI AND NOODLE PRODUCTS Subpart B--Requirements for Specific Standardized Macaroni and Noodle Products Sec. 139.155 Enriched noodle products. FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart D--Special Dietary and Nutritional Additives Sec. 172.345 Folic acid (folacin). FDA PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION Subpart I--Multipurpose Additives Sec. 172.896 Dried yeasts. | |
Physical Properties:
| Appearance: | yellow to yellowish orange crystals (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Melting Point: | 250.00 °C. @ 760.00 mm Hg |
| Flash Point: | 32.00 °F. TCC ( 0.00 °C. ) (est) |
| logP (o/w): | -0.990 (est) |
| Soluble in: | |
| water, 1.6 mg/L @ 25 °C (exp) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intravenous-rat LD50 500 mg/kg Drugs in Japan Vol. -, Pg. 1409, 1995. intravenous-rabbit LD50 410 mg/kg Drugs in Japan Vol. -, Pg. 1409, 1995. oral-mouse LD50 10000 mg/kg Toxicology and Applied Pharmacology. Vol. 23, Pg. 537, 1972. intravenous-mouse LD50 282 mg/kg Toxicology and Applied Pharmacology. Vol. 23, Pg. 537, 1972. intraperitoneal-mouse LD50 85 mg/kg BEHAVIORAL: COMA BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD Experientia. Vol. 41, Pg. 72, 1985. intravenous-guinea pig LD50 120 mg/kg Drugs in Japan Vol. -, Pg. 1409, 1995. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LDLo 200 mg/kg KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: CHANGES IN SPLEEN Proceedings of the Society for Experimental Biology and Medicine. Vol. 71, Pg. 544, 1949. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | special dietary and nutritional additives | ||
| Recommendation for folic acid usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for folic acid flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| European Food Safety Authority (EFSA) reference(s): | |
| Scientific Opinion on the safety and efficacy of folic acid as a feed additive for all animal species View page or View pdf | |
| Scientific Opinion on the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reduced risk of neural tube defects pursuant to Article 14 of Regulation (EC) No 1924/2006 View page or View pdf | |
| Outcome of a public consultation on the Draft Scientific Opinion of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on Dietary Reference Values for folate View page or View pdf | |
| Scientific Opinion on Dietary Reference Values for folate View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| Chemical Carcinogenesis Research Information System: | Search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 59-30-3 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 6037 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 1 |
| (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid | |
| Chemidplus: | 0000059303 |
| EPA/NOAA CAMEO: | hazardous materials |
References:
| (2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 59-30-3 |
| Pubchem (cid): | 6037 |
| Pubchem (sid): | 134972291 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| Metabolomics Database: | Search |
| KEGG (GenomeNet): | C00504 |
| HMDB (The Human Metabolome Database): | HMDB00121 |
| FooDB: | FDB014504 |
| FDA Listing of Food Additive Status: | View |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
| Formulations/Preparations: •5 mg (base) per ml (rx) [folvite (benzyl alcohol 1.5 %)]; 10 mg per ml (rx) •folic acid (folvite) is marketed as oral tablets containing 0.4, 0.8, and 1 mg and as an aqueous solution for injection. tablets of folic acid contain either 0.1, 0.4, 0.8, or 1 mg of pteroylglutamic acid, as an aqueous solution for injection, and in combination with other vitamins and minerals •injectable solutions are prepared by dissolving folic acid in normal sodium bicarbonate solution (which should be sterilized by filtration) or by preparing solutions of the sodium or methylglucamine salt. •lexpec folic acid. syrup containing folic acid 2.5 mg in each 5 ml (recommended diluent, sorbitol solution or sorbitol solution and water, equal parts). •10% feed grade, usp | |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| biological additives |
Occurrence (nature, food, other): note
| almond seed Search Trop Picture | |
| apple fruit Search Trop Picture | |
| apricot fruit Search Trop Picture | |
| asparagus shoot Search Trop Picture | |
| avocado fruit Search Trop Picture | |
| banana fruit Search Trop Picture | |
| barley seed Search Trop Picture | |
| bean asparagus bean seed Search Trop Picture | |
| bean black bean seed Search Trop Picture | |
| bean black bean sprout Search Trop Picture | |
| bean field bean seed Search Trop Picture | |
| bean mung bean seed Search Trop Picture | |
| bean mung bean sprout Search Trop Picture | |
| bean winged bean seed Search Trop Picture | |
| beet root Search Trop Picture | |
| black gram seed Search Trop Picture | |
| blueberry fruit Search Trop Picture | |
| brazil nut Search Trop Picture | |
| broccoli asparagus broccoli leaf Search Trop Picture | |
| brussel sprout leaf Search Trop Picture | |
| cabbage leaf Search Trop Picture | |
| cantaloupe fruit Search Trop Picture | |
| carrot root Search Trop Picture | |
| cashew nut Search Trop Picture | |
| cayenne fruit Search Trop Picture | |
| cherry sour cherry fruit Search Trop Picture | |
| chickpea seed Search Trop Picture | |
| chive leaf Search Trop Picture | |
| chrysanthemum coronarium bud Search Trop Picture | |
| chrysanthemum edible chrysanthemum bud Search Trop Picture | |
| corn fruit Search Trop Picture | |
| corn seed Search Trop Picture | |
| cotton seed Search Trop Picture | |
| cranberry fruit Search Trop Picture | |
| cucumber fruit Search Trop Picture | |
| date palm fruit Search Trop Picture | |
| eggplant fruit Search Trop Picture | |
| endive leaf Search Trop Picture | |
| fenugreek plant Search Trop Picture | |
| gourd calabash gourd fruit Search Trop Picture | |
| grape fruit Search Trop Picture | |
| hazelnut seed Search Trop Picture | |
| jambul fruit Search Trop Picture | |
| kiwi fruit Search Trop Picture | |
| leek wild leek plant Search Trop Picture | |
| lentil seed Search Trop Picture | |
| lentil sprout Search Trop Picture | |
| lettuce leaf Search Trop Picture | |
| mallow jew's mallow leaf Search Trop Picture | |
| mandarin fruit Search Trop Picture | |
| oat seed Search Trop Picture | |
| okra fruit Search Trop Picture | |
| orange fruit Search Trop Picture | |
| parsley plant Search Trop Picture | |
| parsnip root Search Trop Picture | |
| pea black-eyed pea fruit Search Trop Picture | |
| pea black-eyed pea seed Search Trop Picture | |
| pea seed Search Trop Picture | |
| peach fruit Search Trop Picture | |
| peanut seed Search Trop Picture | |
| pear fruit Search Trop Picture | |
| pepper bell pepper fruit Search Trop Picture | |
| peppermint leaf Search Trop Picture | |
| pigweed leaf Search Trop Picture | |
| pistachio nut Search Trop Picture | |
| plum fruit Search Trop Picture | |
| potato sweet potato root Search Trop Picture | |
| potato tuber Search Trop Picture | |
| purslane plant Search Trop Picture | |
| radish root Search Trop Picture | |
| rhubarb plant Search Trop Picture | |
| rosemary leaf Search Trop Picture | |
| sesame seed Search Trop Picture | |
| soursop plant Search Trop Picture | |
| soybean seed Search Trop Picture | |
| spearmint leaf Search Trop Picture | |
| spinach plant Search Trop Picture | |
| squash summer squash fruit Search Trop Picture | |
| tomato fruit Search Trop Picture | |
| walnut english walnut nut Search Trop Picture | |
| watercress plant Search Trop Picture | |
| watermelon fruit Search Trop Picture | |
| wheat seed Search Trop Picture |
Synonyms:
| N-(4-(((2- | amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzo- yl)-L-glutamic acid |
| N-(4-((2- | amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-L-glutamic acid |
| N-(4-((2- | amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-laevo-glutamic acid |
| N-(p-(((2- | amino-4-hydroxy-6-pteridinyl)methyl)amino)benzoyl)-L-glutamic acid |
| N-(4-{[(2- | amino-4-hydroxypteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid |
| N-(4-{[(2- | amino-4-oxo-1,4-dihydro-6-pteridinyl)methyl]amino}benzoyl)-L-glutamic acid |
| (2S)-2-[(4-{[(2- | amino-4-oxo-1,4-dihydro-6-pteridinyl)methyl]amino}benzoyl)amino]pentanedioic acid |
| N-[(4-{[(2- | amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}phenyl)carbonyl]-L-glutamic acid |
| (2S)-2-{[(4-{[(2- | amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}phenyl)carbonyl]amino}pentanedioic acid |
| (2S)-2-[[4-[(2- | amino-4-oxo-1H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid |
| apo-folic | |
| dosfolat B activ | |
| folacin | |
| folate | |
| L- | glutamic acid, N-(4-(((2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzo- yl)- |
| L- | glutamic acid, N-(4-(((2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)- |
| L- | glutamic acid, N-(4-((2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)- |
| glutamic acid, N-(p-(((2-amino-4-hydroxy-6-pteridinyl)methyl)amino)benzoyl)-, L- | |
| glutamic acid, N-(p-(((2-amino-4-hydroxypyrimido(4,5-b)pyrazin-6-yl)methyl)amino)benzoyl)-, L | |
| L- | glutamic acid, N-[4-[[(1,2-dihydro-4-hydroxy-2-imino-6-pteridinyl)methyl]amino]benzoyl]- |
| L- | glutamic acid, N-[4-[[(2-amino-1,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]- |
| L- | glutamic acid, N-[4-[[(2-amino-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]- |
| L- | glutamic acid, N-[4-[[(2-amino-4,8-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]- |
| glutamic acid, N-[p-[[(2-amino-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]-, l- | |
| glutamic acid, pteroyl-, L- | |
| novo-folacid | |
| pteroyl glutamic acid | |
| pteroyl-L-glutamic acid | |
| N- | pteroyl-L-glutamic acid |
| pteroyl-L-monoglutamic acid | |
| L- | pteroylglutamic acid |
| vitamin M |








