Articles:
L-lysine monoacetate
Notes:
None found
| CAS Number: | 57282-49-2 | 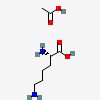 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 260-664-4 | |
| FDA UNII: | TTL6G7LIWZ | |
| MDL: | MFCD00039069 | |
| Molecular Weight: | 206.24226000 | |
| Formula: | C8 H18 N2 O4 | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: special dietary and nutritional additives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Appearance: | white powder (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Boiling Point: | 311.00 to 313.00 °C. @ 760.00 mm Hg |
| Vapor Pressure: | 0.000123 mmHg @ 25.00 °C. (est) |
| Flash Point: | 287.00 °F. TCC ( 141.67 °C. ) |
| logP (o/w): | -1.036 (est) |
| Soluble in: | |
| water, 2.7e+005 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| None found |
Suppliers:
| Ajinomoto USA |
| L-Lysine Acetate
Flavor: characteristic L-lysine is classified as an essential amino acid for humans and must be supplied in the diet. The male adult’s daily requirement is 12 mg per kg of body weight. It is extremely rare for a diet to contain insufficient quantities of lysine. Vegetarians who follow a macrobiotic diet and athletes who exercise vigorously on a frequent basis must take care to obtain sufficient L-lysine. Beans, peas and lentils are the best source of lysine. |
| BOC Sciences |
| For experimental / research use only. |
| L-Lysine acetate salt 98.0-102.0% (Assay, dried basis) |
| Matrix Scientific |
| For experimental / research use only. |
| L-Lysine acetate, 95+% |
| Penta International |
| L-LYSINE MONOHYDRATE |
| Sigma-Aldrich: Sigma |
| For experimental / research use only. |
| L-Lysine acetate salt ≥98% (TLC) |
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| None - None found. | |
|
S 02 - Keep out of the reach of children. S 22 - Do not breath dust. S 24/25 - Avoid contact with skin and eyes. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
intraperitoneal-rat LD50 3700 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 1253, 1981. oral-mouse LD50 13400 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 1253, 1981. intravenous-mouse LD50 3700 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 1253, 1981. intraperitoneal-mouse LD50 5100 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 12, Pg. 933, 1981. intravenous-rat LD50 2850 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 1253, 1981. oral-rat LD50 11400 mg/kg Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 23, Pg. 1253, 1981. | |
| Dermal Toxicity: | |
|
subcutaneous-mouse LD50 5800 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 12, Pg. 933, 1981. subcutaneous-rat LD50 4000 mg/kg Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 12, Pg. 933, 1981. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | special dietary and nutritional additives | ||
| Recommendation for laevo-lysine acetate usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for laevo-lysine acetate flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 57282-49-2 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 104152 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 2 |
| acetic acid; (2S)-2,6-diaminohexanoic acid | |
| Chemidplus: | 0057282492 |
| RTECS: | OL5644200 for cas# 57282-49-2 |
References:
| acetic acid; (2S)-2,6-diaminohexanoic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 57282-49-2 |
| Pubchem (cid): | 104152 |
| Pubchem (sid): | 135061173 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEMBL: | View |
| KEGG (GenomeNet): | D02278 |
| HMDB (The Human Metabolome Database): | Search |
| FDA Listing of Food Additive Status: | View |
| ChemSpider: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| not found in nature |
Synonyms:
| acetic acid; (2S)-2,6-diaminohexanoic acid | |
| L- | lysine acetate |
| L- | lysine monoacetate |
| laevo- | lysine monoacetate |
| L- | lysine monohydrate |
| L- | lysine, acetate |
| L- | lysine, monoacetate |
| L- | lysinemonoacetate |

