Articles:
undec-10-enoic acid
Notes:
Flavouring ingredient
| CAS Number: | 112-38-9 | 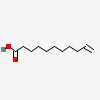 3D/inchi 3D/inchi
|
| Other(deleted CASRN): | 949900-17-8 | |
| ECHA EINECS - REACH Pre-Reg: | 203-965-8 | |
| FDA UNII: | K3D86KJ24N | |
| Nikkaji Web: | J5.125J | |
| Beilstein Number: | 1762631 | |
| MDL: | MFCD00004442 | |
| CoE Number: | 689 | |
| XlogP3: | 3.90 (est) | |
| Molecular Weight: | 184.27880000 | |
| Formula: | C11 H20 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: flavor and fragrance agents
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Perfumer and Flavorist: | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
| JECFA Food Flavoring: | 331 10-undecenoic acid |
| DG SANTE Food Flavourings: | 08.039 undec-10-enoic acid |
| FEMA Number: | 3247 10-undecenoic acid |
| FDA: | No longer provide for the use of these seven synthetic flavoring substances |
| FDA Mainterm (SATF): | 112-38-9 ; 10-UNDECENOIC ACID |
Physical Properties:
| Appearance: | colorless white solid (est) |
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.91000 to 0.91300 @ 25.00 °C. |
| Pounds per Gallon - (est).: | 7.572 to 7.597 |
| Refractive Index: | 1.44700 to 1.44800 @ 20.00 °C. |
| Melting Point: | 25.00 to 27.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 137.00 °C. @ 2.00 mm Hg |
| Flash Point: | 300.00 °F. TCC ( 148.89 °C. ) |
| logP (o/w): | 3.860 |
| Soluble in: | |
| alcohol | |
| water, 73.7 mg/L @ 30 °C (exp) | |
| Insoluble in: | |
| water | |
Organoleptic Properties:
| Odor Description: at 100.00 %. | sweet woody |
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data2 |
| CosIng: | cosmetic data |
| Cosmetic Uses: |
cleansing agents preservatives surfactants surfactant - emulsifying |
Suppliers:
Safety Information:
| Preferred SDS: View | |
| European information : | |
| Most important hazard(s): | |
| Xi - Irritant | |
|
R 36/37/38 - Irritating to eyes, respiratory system, and skin. R 43 - May cause sensitisation by skin contact. S 02 - Keep out of the reach of children. S 24/25 - Avoid contact with skin and eyes. S 26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 - Wear suitable gloves and eye/face protection. | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
|
gavage-mouse LD50 8150 mg/kg (Newell et al., 1949) oral-mouse LD50 2300-6600 mg/kg (Tislow et al., 1950) oral-rat LD50 2500 mg/kg (Tislow et al., 1950) intraperitoneal-mouse LD50 960 mg/kg Journal of Investigative Dermatology. Vol. 13, Pg. 145, 1949. oral-mouse LD50 8150 mg/kg BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT Journal of Investigative Dermatology. Vol. 13, Pg. 145, 1949. oral-rat LD50 2500 mg/kg "Pesticide Index," Frear, E.H., ed., State College, PA, College Science Pub., 1969Vol. 4, Pg. 386, 1969. | |
| Dermal Toxicity: | |
|
skin-guinea pig LD50 50 mg/kg "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4954, 1982. | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | flavor and fragrance agents | ||
| RIFM Fragrance Material Safety Assessment: Search | |||
| IFRA Code of Practice Notification of the 49th Amendment to the IFRA Code of Practice | |||
| Recommendation for 10-undecenoic acid usage levels up to: | |||
| 0.2000 % in the fragrance concentrate. | |||
| Maximised Survey-derived Daily Intakes (MSDI-EU): | 26.00 (μg/capita/day) | ||
| Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS). | |||
| The Expert Panel also publishes separate extensive reviews of scientific information on all FEMA GRAS flavoring substances and can be found at FEMA Flavor Ingredient Library | |||
| publication number: 4 | |||
| Click here to view publication 4 | |||
| average usual ppm | average maximum ppm | ||
| baked goods: | - | 0.50000 | |
| beverages(nonalcoholic): | - | 0.50000 | |
| beverages(alcoholic): | - | - | |
| breakfast cereal: | - | - | |
| cheese: | - | - | |
| chewing gum: | - | - | |
| condiments / relishes: | - | - | |
| confectionery froastings: | - | - | |
| egg products: | - | - | |
| fats / oils: | - | - | |
| fish products: | - | - | |
| frozen dairy: | - | 0.50000 | |
| fruit ices: | - | 0.50000 | |
| gelatins / puddings: | - | - | |
| granulated sugar: | - | - | |
| gravies: | - | - | |
| hard candy: | - | 0.50000 | |
| imitation dairy: | - | - | |
| instant coffee / tea: | - | - | |
| jams / jellies: | - | - | |
| meat products: | - | - | |
| milk products: | - | - | |
| nut products: | - | - | |
| other grains: | - | - | |
| poultry: | - | - | |
| processed fruits: | - | - | |
| processed vegetables: | - | - | |
| reconstituted vegetables: | - | - | |
| seasonings / flavors: | - | - | |
| snack foods: | - | - | |
| soft candy: | - | - | |
| soups: | - | - | |
| sugar substitutes: | - | - | |
| sweet sauces: | - | - | |
Safety References:
| European Food Safety Athority(EFSA): | Flavor usage levels; Subacute, Subchronic, Chronic and Carcinogenicity Studies; Developmental / Reproductive Toxicity Studies; Genotoxicity Studies... |
| European Food Safety Authority (EFSA) reference(s): | |
| Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Flavouring Group Evaluation 6 (FGE.06): Straight-and branched-chain aliphatic unsatured primary alcohols, aldehydes, carboxylic acids, and esters from chemical groups 1 and 4 View page or View pdf | |
| Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to Flavouring Group Evaluation 5: Esters of 23 branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and 24 branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 View page or View pdf | |
| Flavouring Group Evaluation 6, Revision 1 (FGE.06Rev1) - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 5, Revision 1 (FGE.05Rev1):Esters of branched- and straight-chain aliphatic saturated primary alcohols and of one secondary alcohol, and branched- and straight-chain unsaturated carboxylic acids from chemical groups 1, 2, and 5 (Commission Regulation (EC) No 1565/2000 of 18 July 2000) [1] - Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) View page or View pdf | |
| Flavouring Group Evaluation 5, Revision 2 (FGE.05Rev2): Branched- and straight-chain unsaturated carboxylic acids and esters of these with aliphatic saturated alcohols from chemical groups 1, 2, 3 and 5 View page or View pdf | |
| EPI System: | View |
| ClinicalTrials.gov: | search |
| Daily Med: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA Substance Registry Services (TSCA): | 112-38-9 |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 5634 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WISER: | UN 3261 |
| WGK Germany: | 1 |
| undec-10-enoic acid | |
| Chemidplus: | 0000112389 |
| RTECS: | YQ2975000 for cas# 112-38-9 |
References:
| undec-10-enoic acid | |
| NIST Chemistry WebBook: | Search Inchi |
| Canada Domestic Sub. List: | 112-38-9 |
| Pubchem (cid): | 5634 |
| Pubchem (sid): | 134973166 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| FDA Substances Added to Food (formerly EAFUS): | View |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C13910 |
| HMDB (The Human Metabolome Database): | HMDB33724 |
| FooDB: | FDB011844 |
| Export Tariff Code: | 2916.19.3000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| jasmin | FR | |
| oriental | FR | |
| woody | FR |
Occurrence (nature, food, other): note
| castor oil Search Trop Picture | |
| elder black elder seed Search Trop Picture |
Synonyms:
| declid | |
| 10- | hendecenoic acid |
| omega- | hendecenoic acid |
| renselin | |
| sevinon | |
| undec-10-enoic acid | |
| undecenoic acid | |
| omega- | undecenoic acid |
| undecyl-10-enic acid | |
| undecylenic acid | |
| 10- | undecylenic acid |
| undecylenic acid natural | |
| undecylenic acid U.S.P. grade |













