Articles:
lup-20(30)-ene-3beta,28-diol
Notes:
Constit. of Corylus avellana (filbert) and Vicia faba
Betulin (lup-20(29)-ene-3?,28-diol) is an abundant naturally occurring triterpene. It is commonly isolated from the bark of birch trees and forms up to 30% of the dry weight of the extractive. The purpose of the compound in the bark is not known. It can be converted to betulinic acid (the alcohol group replaced by a carboxylic acid group), which is biologically more active than betulin itself.; Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28. Triterpenoid is a byproduct from squalene 2,3-oxide decomposition. Squalene is a precursor to sterol biosynthesis. Terpenes are derived biosynthetically from units of isoprene, which has the molecular formula C5H8. The basic molecular formulas of terpenes are multiples of that, (C5H8)n where n is the number of linked isoprene units. This is called the isoprene rule or the C5 rule. The isoprene units may be linked together "head to tail" to form linear chains or they may can be arranged to form rings. One can consider the isoprene unit as one of nature's preferred building blocks. [HMDB]
| CAS Number: | 473-98-3 | 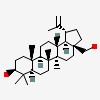 3D/inchi 3D/inchi
|
| ECHA EINECS - REACH Pre-Reg: | 207-475-5 | |
| FDA UNII: | 6W70HN7X7O | |
| Nikkaji Web: | J5.959E | |
| MDL: | MFCD00016802 | |
| XlogP3-AA: | 8.30 (est) | |
| Molecular Weight: | 442.72690000 | |
| Formula: | C30 H50 O2 | |
| BioActivity Summary: | listing | |
| NMR Predictor: | Predict (works with chrome or firefox) | |
Category: natural substances and extractives
US / EU / FDA / JECFA / FEMA / FLAVIS / Scholar / Patent Information:
| Google Scholar: | Search |
| Google Books: | Search |
| Google Scholar: with word "volatile" | Search |
| Google Scholar: with word "flavor" | Search |
| Google Scholar: with word "odor" | Search |
| Google Patents: | Search |
| US Patents: | Search |
| EU Patents: | Search |
| Pubchem Patents: | Search |
| PubMed: | Search |
| NCBI: | Search |
Physical Properties:
| Assay: | 95.00 to 100.00 % |
| Food Chemicals Codex Listed: | No |
| Specific Gravity: | 0.97500 @ 25.00 °C. |
| Refractive Index: | 1.50179 @ 20.00 °C. |
| Melting Point: | 147.00 to 148.00 °C. @ 760.00 mm Hg |
| Boiling Point: | 284.00 to 288.00 °C. @ 743.00 mm Hg |
| Boiling Point: | 138.00 to 140.00 °C. @ 4.00 mm Hg |
| Flash Point: | 412.00 °F. TCC ( 210.90 °C. ) (est) |
| logP (o/w): | 8.607 (est) |
| Soluble in: | |
| water, 0.0001726 mg/L @ 25 °C (est) | |
Organoleptic Properties:
| Odor and/or flavor descriptions from others (if found). | |
Cosmetic Information:
| CosIng: | cosmetic data |
| Cosmetic Uses: |
skin conditioning |
Suppliers:
| Alfa Biotechnology |
| For experimental / research use only. |
| Betulin 98% |
| BOC Sciences |
| For experimental / research use only. |
| Betulin >98%
Odor: characteristic Use: Betulin can be found in the barks of Betula alba L. Its nanoparticles powder show an excellent hypoglycemic effect compared with raw Betulin. Betulin alleviated LPS-induced acute lung injury. |
| EMD Millipore |
| For experimental / research use only. |
| SREBP Processing Inhibitor, Betulin |
| ExtraSynthese |
| For experimental / research use only. |
| Betulin (HPLC) ≥98% |
| Glentham Life Sciences |
| Betulin |
| Santa Cruz Biotechnology |
| For experimental / research use only. |
| Betulin |
| Sigma-Aldrich: Aldrich |
| For experimental / research use only. |
| Betulin ≥98% |
| Skyherb Technologies |
| Betulin 98% |
| Xian Yuensun Biological Technology |
| Betulin, Dioscin 6% - 98% |
Safety Information:
| Preferred SDS: View | |
| Hazards identification | |
| Classification of the substance or mixture | |
| GHS Classification in accordance with 29 CFR 1910 (OSHA HCS) | |
| None found. | |
| GHS Label elements, including precautionary statements | |
| Pictogram | |
| Hazard statement(s) | |
| None found. | |
| Precautionary statement(s) | |
| None found. | |
| Oral/Parenteral Toxicity: | |
| Not determined | |
| Dermal Toxicity: | |
| Not determined | |
| Inhalation Toxicity: | |
| Not determined | |
Safety in Use Information:
| Category: | natural substances and extractives | ||
| Recommendation for betulin usage levels up to: | |||
| not for fragrance use. | |||
| Recommendation for betulin flavor usage levels up to: | |||
| not for flavor use. | |||
Safety References:
| EPI System: | View |
| Daily Med: | search |
| AIDS Citations: | Search |
| Cancer Citations: | Search |
| Toxicology Citations: | Search |
| EPA ACToR: | Toxicology Data |
| EPA Substance Registry Services (SRS): | Registry |
| Laboratory Chemical Safety Summary : | 72326 |
| National Institute of Allergy and Infectious Diseases: | Data |
| WGK Germany: | 3 |
| Chemidplus: | 0000473983 |
References:
| NIST Chemistry WebBook: | Search Inchi |
| Pubchem (cid): | 72326 |
| Pubchem (sid): | 135029171 |
Other Information:
| (IUPAC): | Atomic Weights of the Elements 2011 (pdf) |
| Videos: | The Periodic Table of Videos |
| tgsc: | Atomic Weights use for this web site |
| (IUPAC): | Periodic Table of the Elements |
| CHEBI: | View |
| CHEMBL: | View |
| KEGG (GenomeNet): | C08618 |
| HMDB (The Human Metabolome Database): | HMDB36838 |
| FooDB: | FDB023363 |
| Export Tariff Code: | 2906.19.0000 |
| VCF-Online: | VCF Volatile Compounds in Food |
| ChemSpider: | View |
| Wikipedia: | View |
Potential Blenders and core components note
| None Found | ||
Potential Uses:
| None Found |
Occurrence (nature, food, other): note
| bean fava bean root Search Trop Picture | |
| betula alba l. leaves Search Trop Picture | |
| broadbean root Search Trop Picture | |
| chestnut sweet chestnut leaf Search Trop Picture | |
| chicory Search Trop Picture | |
| elder black elder bark Search Trop Picture | |
| hazelnut bark Search Trop Picture | |
| mangosteen leaf Search Trop Picture | |
| nance leaf Search Trop Picture | |
| persimmon Search Trop Picture | |
| persimmon american persimmon Search Trop Picture | |
| persimmon oriental persimmon root Search Trop Picture | |
| persimmon root Search Trop Picture | |
| persimmon stem bark Search Trop Picture | |
| rosemary Search Trop Picture | |
| rosemary plant Search Trop Picture | |
| rosemary shoot Search Trop Picture | |
| sage leaf Search Trop Picture | |
| walnut english walnut bark Search Trop Picture |
Synonyms:
| betuline | |
| betulinic alcohol | |
| betulinol | |
| betulol | |
| (3beta)- | lup-20(29)-ene-3,28-diol |
| 3b- | lup-20(29)-ene-3,28-diol |
| lup-20(29)-ene-3,28-diol, (3b)- | |
| lup-20(29)-ene-3b,28-diol | |
| lup-20(30)-ene-3b,28-diol | |
| lup-20(30)-ene-3beta,28-diol | |
| messagenin | |
| triterpenoid | |
| trochol |

